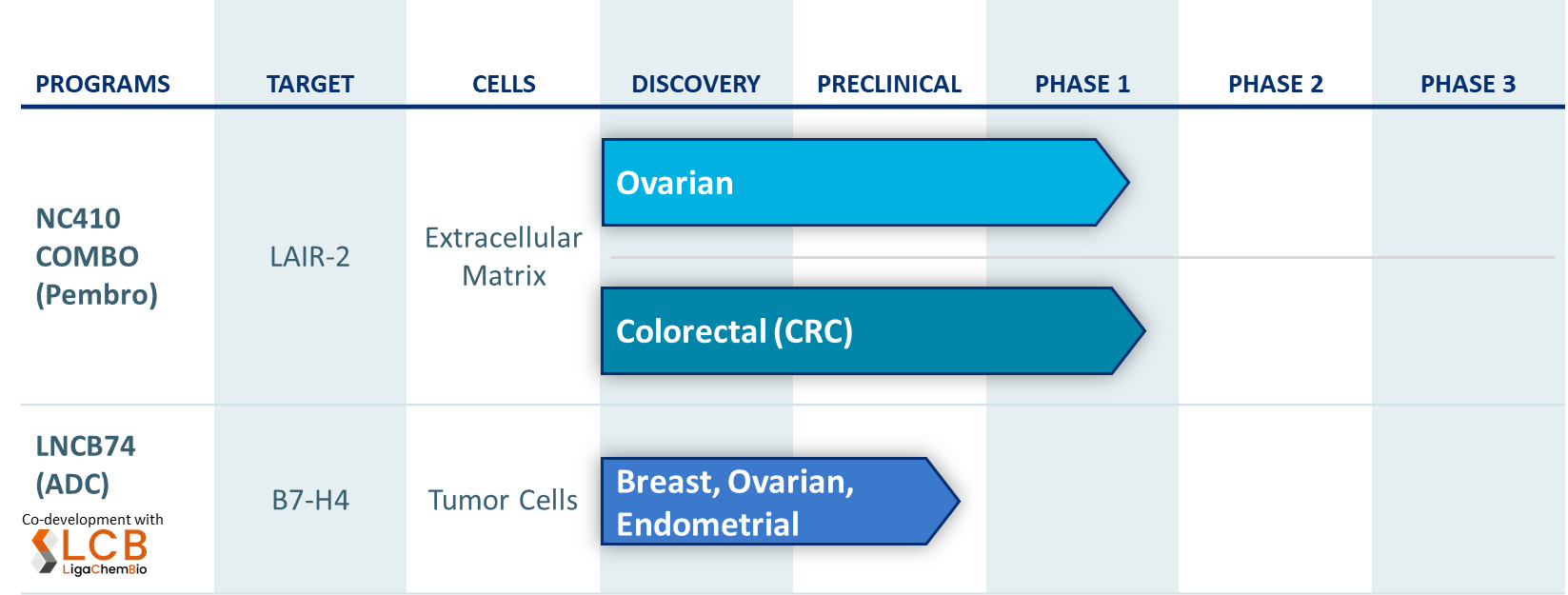
NC410
NC410 is a first-in-class immunomedicine designed to block immune suppression mediated by LAIR-1, an immunomodulatory receptor expressed on a variety of immune cells including T cells and myeloid cells. In non-clinical models and early-stage monotherapy clinical studies, we have demonstrated that NC410 can remodel collagen in the extracellular matrix (ECM), the tissue matrix surrounding the tumor, which enhances T cell infiltration into the tumor. Elevated collagen levels in the ECM are associated with resistance to PD-1 and PD-L1 therapies. Our translational work has shown that NC410 blocks the interaction of LAIR-1 with its binding partners, thereby promoting T cell function and immune cell mediated activity to contribute to restoring anti-tumor activity. Consistent with our strategy, we believe NC410 has the potential to address the needs of patients who are not adequately addressed by currently available therapies.
NC410 is currently in a Ph1/2 trial in combination with pembrolizumab for the treatment of patients not eligible or refractory to PD-(L)1 therapeutics. NC410 combo shows evidence of clinical activity in hard-to-treat cancers: (i) 3 partial responses out 7 patients in PD-(L)1 naive ovarian cancer and (ii) 2 partial responses out of 19 patients in MSS/MSI-L colorectal cancer without liver metastasis.
Clinical Trial NCT05572684.
LNCB74
LNCB74 is an antibody-drug conjugate that binds specifically to B7-H4, a validated target on tumors. B7-H4 protein is expressed on multiple tumor types, including many cancers specific to women (breast, ovarian and endometrial). LNCB74 has been specifically designed to reduce toxicity while improving tumor killing. Our research showed LNCB74 has potent antitumor activity in in animal models, including models with low expression of B7-H4. LNCB74 is advancing towards an IND by end of 2024 with on-going GMP manufacturing and initiation of GLP tox studies.
LNCB74 is advancing towards an IND by end of 2024 with on-going GMP manufacturing and initiation of GLP tox studies.
Expanded Access Policy
NextCure Expanded Access Policy can be viewed here.

